Because distillations from aqueous solutions are energy intensive, pervaporation could be an energy savings separation alternative, as much less energy is potentially required compared to evaporation methods. The permeability of hydrophobic membranes towards butanols involves transfer of polar molecules (the alcohols) from a polar medium (water) into a nonpolar environment (the membrane). Moreover, selective pervaporation of organics from aqueous-organic solution requires elastomeric membranes. As such, a hydrophobic elastomeric silicone (PDMS) membrane is ideal for removing butanol from water.
How to separate butanol from water using a silicone membrane
WARNING. Our standard PermSelect® membrane modules have not been optimized for pilot scale, or production pervaporation of butanol, and may not endure to the user's satisfaction. Please contact us to discuss changes in module design which may improve membrane endurance.
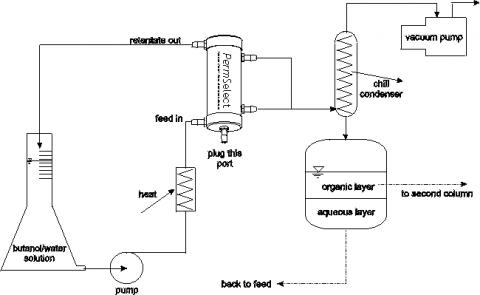
Separating butanols from water by pervaporation is simple and straightforward as illustrated in the figure below. Since the permeability of butanons in silicone (PDMS) is much higher than that of water, it is possible to achieve high separation factors, even with highly diluted broths. The water-butanol mixture is supplied to one side of the silicone membrane through the feed port. A vacuum is applied to the other side of the membrane through the permeate ports. As the mixture flows through membrane module, butanol and water vapor permeate the membrane toward the vacuum side. These vapors can be condensed resulting in a highly concentrated butanol-water mixture, which automatically separates into two layers. The bottom water layer can be returned to the membrane module for additional pervaporation and the top organic layer can be sent to a second column for additional purification.
PermSelect's silicone hollow fiber membrane configuration provides many advantages over the spiral wound format, including up to ten times higher membrane area density per volume, no porous substrate to impede gas transfer or limit compatibility, and substantially lower cost per unit area. Moreover, our patented forming process allows for incorporating molecular sieves such as zeolites to form mixed matrix membranes tailored for specific separation processes.
While MedArray currently provides modules for laboratory and pilot testing, we are actively seeking strategic partners with well established presence in this field to collaborate on scaling-up and developing products incorporating our membrane technology. If your organization is interested in providing butanol separating solutions using our patented technology, Contact us or call us at +1 (734) 769-1066 x21 to discuss opportunities.
Pervaporation of Butanol using a PermSelect® Silicone Membrane Module Several researchers have studied and demonstrated the feasibility of separating butanol from water using silicone membranes as described in the following articles:
Pervaporation of n-Butanol/Water and Iso-Butanol/Water Solutions using a PDMS Hollow-Fiber Membrane Module Submitted for poster presentation NAMS 2012, June 9-13, 2012, New Orleans, LA. Peiyuan Li, Ph.D. and Patrick Montoya, Ph.D., MedArray, Inc.
Abstract The depleting supply and rising cost of crude oil has recently created extensive interest in converting biomass and agricultural wastes for energy production. Butanol produced by fermentation of biomass (Biobutanol) has been proposed as a substitute for diesel fuel and gasoline; it is considered as a potential biofuel with superior properties when compared to ethanol. Butanol has a higher energy density, and lower vapor pressure than ethanol and gasoline. Butanol can be used as a replacement for gasoline gallon for gallon, and has been demonstrated to work in vehicles designed for use with gasoline without modification. Ethanol can only be used as an additive to gasoline up to about 85% and then only after modifications to the engine.
Bio-butanol can be produced by fermentation, however, the concentration of n-butanol (the most common butanol isomer produced in fermentation) is lower than that achieved in ethanol fermentation. The feedstocks are the same as for ethanol: energy crops such as sugar beets, sugar cane, corn grain, wheat and cassava, prospective non-food energy crops such as switchgrass, as well as agricultural byproducts such as straw and corn stalks.
Iso-butanol, a butanol isomer with branched chain structure, gives a higher octane number than the isomeric n-butanol. Iso-butanol is also produced naturally during the fermentation of carbohydrates and may also be a byproduct of the decay of organic matter. Iso-butanol, along with other low molecular weight alcohols can also be produced by some engineered microorganisms such as corynebacterium. In recent years, production of iso-butanol has also been investigated using engineered microbial strains that can reach higher concentrations during aerobic cultivations.
Butanol, unlike ethanol has a much lower solubility in water and thus requires much less energy for separating from water. N-butanol/water and iso-butanol/water solutions are typically separated with two distillation columns as both form heterogeneous azeotropes in the distillation processes. Typical starting concentrations for enriched n-butanol/water and iso-butanol/water mixtures are smaller than 9 wt%.
Because distillations from aqueous solutions are energy intensive, pervaporation could be an energy savings separation alternative, as much less energy is potentially required compared to evaporation methods. The permeability of hydrophobic membranes towards butanols involves transfer of polar molecules (the alcohols) from a polar medium (water) into a nonpolar environment (the membrane). Moreover, selective pervaporation of organics from aqueous-organic solution requires elastomeric membranes. As such, a hydrophobic elastomeric membrane material such as PDMS is suitable for removing butanol from water as previously demonstrated by other researchers.
Polydimethylsiloxane (PDMS) silicone is one of the most widely known membrane materials because of its high permeability to vapors and gases. It has been widely used in spiral wound membrane modules as well as skinned on porous hollow fibers in applications ranging from pervaporation and liquid contacting to gas separation. However, it has not been commonly used in a dense homogeneous hollow fiber format, mainly because of the difficulty and cost in forming PDMS into suitably sized dense hollow fibers. MedArray has developed a low cost method for forming dense, homogeneous PDMS hollow fibers and has packaged the hollow fibers in membrane modules or various sizes with membrane area densities as high as 5,000 m2/m3.
The work presented here is directed toward demonstrating the efficacy of MedArray’s PDMS membrane hollow fiber modules to separate butanols from water by pervaporation. Pervaporation could replace the first distillation column, thus requiring much less energy for separation. The butanol/water solutions can be fed to a membrane module, instead of the first distillation column. Butanol (and some water) will permeate the membrane while butanol depleted water (retentate) recirculates continuously through the module. The permeate consists of butanol and water vapors which when condensed, automatically separate into two liquid layers (aqueous and organic) because of the poor solubility of butanol in water. The liquid layers are contained in a separator decanter. The enriched water phase from the bottom of the separator goes back into the feed side of the membrane module, while the enriched butanol phase on top is decanted and fed to a distillation column. Finally, highly purified butanol emerges from the bottom of the column while the azeotrope exits the top of the column, is condensed, and then fed back to the separator.
Methods
The membrane modules used were MedArray’s PDMSXA-8300 containing 10600 PDMS hollow fibers in a 3.2 mm diameter housing, providing an exposed length of 8.3 cm with effective surface area 0.56 m2.The dimensions of the hollow fiber are as follows: outside diameter 300 microns, wall thickness 56 microns. These modules were tested while simulating a series of butanol separation applications.
2.8 kg of a mixture of n-butanol/water or iso-butanol/water was filled into a flask with a graduated volume scale at the neck. A centrifugal pump circulated the mixture through the membrane module as the feed through the tube side. The permeate was driven across the membrane by vacuum or a sweep gas. The initial and final concentrations of the feed were determined by GC.
The membrane module performance can be characterized in terms of permeation flux (J) and separation factor (a) defined as:
J = Q/(At)
a = [cb/cw]Permeate / [cb/cw]Feed
where Q is the mass of permeate collected over a time interval t, A is the effective membrane area for permeation; cb and cw are the mass fractions of butanol and water, respectively.
Results
| Test | Butanol Isomer | Initial Feed Conc. wt% | Temp °C | Sweep or Vacuum | Final Feed Conc. wt% | Flux J g/hr/m2 Butanol | Flux J g/hr/m2 water | Separation Factor a |
| 1 | Iso-butanol | 7.5 | 42 | vacuum | 1.6 | 71.5 | 67.8 | 22 |
| 2 | Iso-butanol | 7.5 | 27 | vacuum | 3.6 | 42.2 | 29 | 24.6 |
| 3 | Iso-butanol | 7.5 | 26 | N2 sweep | 5.4 | 23.1 | 12.1 | 27.8 |
| 4 | 1-butanol | 7.5 | 27 | vacuum | 3.3 | 45.1 | 32.9 | 23.7 |
| 5 | 1-butanol | 6.3 | 25 | N2 sweep | 5.1 | 13.3 | 9.4 | 23.4 |
| 6 | 1-butanol | 7.5 | 25 | air sweep | 6.3 | 14 | 11.4 | 16.6 |
Conclusions
Based on these results, we conclude that PDMS hollow fiber membrane modules can be used effectively in iso-butanol/water and 1-butanol/water separation processes. At higher temperature, permeation flux is higher, but the separation factor is lower.
Pervaporation of isomeric butanols Journal of Membrane Science, 54 (1990) l-12K.W. Biiddeker, G. Bengtson and H. Pingel.
Abstract The pervaporation behavior of aqueous butanols and related alcohols (ethanol, benzyl alcohol) through elastomeric membranes is analyzed and compared with other relevant means of alcoholwater separation. Pervaporation, like evaporation, is governed by the thermodynamic condition of the aqueous feed solutions; however, pervaporative enrichment increases with decreasing vapor pressure (increasing boiling point) of the alcohols. Butanol diffusivity in the membrane polymer is additionally influenced by structural branching of the isomers, as are rejection by reverse osmosis and adsorption by zeolites.
Pervaporative separation of n-butanol from dilute aqueous solutions using silicalite-filled poly(dimethyl siloxane) membranes Journal of Membrane Science, Volume 339, Issues 1–2, 1 September 2009, Pages 120–125. Elsayed A. Fouad1, Xianshe Feng
Abstract Pervaporative separation of n-butanol from dilute aqueous solutions (<0.5 wt%) using a silicalite-filled poly(dimethyl siloxane) composite membrane was investigated. The effects of operating conditions (e.g., feed composition, temperature) on the permeation flux, separation factor and pervaporation separation index were evaluated. It was shown that at a given temperature, water flux increased almost linearly with an increase in feed butanol concentration, whereas the butanol flux increased in a concave fashion due to silicalite fillers that have a strong affinity to butanol molecules. Consequently, the permeate butanol concentration initially increased and then gradually leveled off when the feed butanol concentration was high enough, and the leveling off started to occur at a lower butanol concentration at a higher temperature. The temperature dependence of permeation flux followed a typical Arrhenius relation, and a variation in temperature would increase or decrease the membrane selectivity, depending on feed butanol concentration. These results are especially important for potential use of the membrane for in situ butanol extraction from fermentation where butanol becomes inhibitory at a low concentration of 4–6 g/L.