Volatile organic compounds (VOC’s) and other analytes in air or liquid streams pose a challenge for separation approaches. Sensing, removing, recovering and recycling such compounds and solvents may be desirable economically, as many have significant market value or may be reused in the process. Decreased discharge and lower consumption of chemicals reduces operating costs. In addition, increasingly stringent regulatory requirements make sensing and recovery of VOC’s more and more important.
Using a silicone (PDMS) membrane module to remove and recover volatile organic compounds (VOC). Note that for VOC sensing and detection, the permeate with the concentrated analyte would go the analyzer such as a GC. Silicone (PDMS) membranes can be used for the separation and recovery of VOC’s as well as for VOC sensing due to the unique characteristics of the material. Polydimethylsiloxane (PDMS) is the most commonly used membrane material for the separation of organic vapors from permanent gases and liquids. Certain VOC’s permeate the membrane very well, and can be effectively detected and removed from the feed stream. In a pervaporation system, the VOC vapors are permeated from the feed liquid stream; in a vapor recovery system, a vacuum or an inert sweep gas is used to create the driving force across the membrane.
How to detect, remove, and recover VOCs using PermSelect® membrane modules?
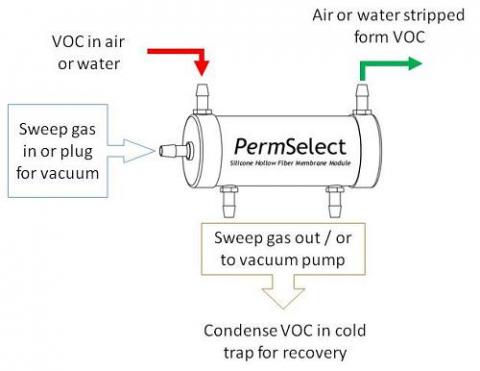
VOC sensing, stripping and recovery using PermSelect® membrane modules is straightforward as illustrated in the figure above. Air or water containing traces of organic volatile compounds is supplied to one side of the silicone membrane through the feed port of the membrane module. A vacuum or an inert sweep gas is supplied to the opposite side of the membrane through the permeate ports of the module. The VOCs permeate the membrane from the feed to the vacuum, thereby stripping the feed from the VOCs, and exiting the module VOC free. The VOC's in the permeate can be used for detection in a GC, or condensed for recovery upstream from the vacuum pump. Consideration must me made to the pressure, temperature, and concentration of the particular VOCs to be removed. Contact us to discuss your particular VOC removal and recovery application.
The following articles describe using silicone membranes to effectively separate organic compounds from air and water to control pollution, recover solvents, sense VOC's, and to detect pollutants. Many of these applications have not been widely attractive because of the high cost per unit area of spiral wound, or composite hollow fiber membrane modules. Our platform technology has enabled the production of lower cost homogeneous silicone hollow fiber membrane modules which could provide for commercially attractive implementation.
Determination of fuel ethers in water by membrane extraction ion mobility spectrometry Talanta, Volume 106, 15 March 2013, Pages 448–453 S. Holopainen, et al.
Abstract Fuel oxygenates are environmentally detrimental compounds due to their rapid migration to groundwater. Fuel oxygenates have been reported to cause taste and odour problems in drinking water, and they also have long-term health effects. Feasible analytical methods are required to observe the presence of fuel oxygenates in drinking and natural water. The authors studied ion mobility spectrometry (IMS) to determinate isomeric fuel ether oxygenates; ethyl tert-butyl ether (ETBE), diisopropyl ether (DIPE), and tert-amyl methyl ether (TAME), separated from aqueous matrices with a pervaporation membrane module. Methyl tert-butyl ether (MTBE) was also membrane extracted and detected with IMS. The authors demonstrated that fuel ethers (MTBE, ETBE, DIPE, and TAME) can be quantified at μg/L level with membrane extraction IMS. A membrane extraction module coupled to IMS is a time and cost effective analysis method because sampling can be performed in a single procedure and from different natural water matrices within a few minutes. Consequently, IMS combined with membrane extraction is suitable not only for waterworks and other online applications but also in the field monitoring the quality of drinking and natural water.
Vapour permeation for the recovery of organic solvents from waste air streams: separation capacities and process optimization Journal of Membrane Science, Volume 113, Issue 2, 15 May 1996, Pages 313–322 M. Leemann, et al.
Abstract Vapour permeation is a potentially suitable technology for the recovery of organic solvents from waste air streams. New solvent stable capillary membrane modules that are currently emerging on the market provide large membrane areas for an acceptable price and enhance the competitiveness of this process. Most membranes used in vapour permeation are silicone coated composites. Polydimethylsiloxane (PDMS) provides good separation capabilities and is highly permeable. The permeabilities for solvents and permanent gases show an inverse temperature dependence due to the different enthalpies of sorption and diffusion. Selectivities of silicone coated composite membranes are lower than that of pure PDMS but still high enough to enable a high degree of enrichment. The investigation of systems with two solvent components in air shows that selectivities and permeabilities are only slightly lower than in the case when only one solvent component is present. Coupling effects like preferential sorption are, therefore, not very strong. The experimental results have been used as basis for an economical process optimization. Comparison with other waste air cleaning technologies shows that in the range of medium to high solvent concentrations and low to medium feed volume fluxes vapour permeation can be an economical alternative to the conventional processes.
Effect of Operating Conditions on Pervaporative Removal of Styrene from Aqueous Solutions American Journal of Scientific Research ISSN 1450-223X Issue 37(2011), pp.123-131 Majid Aliabadi, et al.
Abstract The contamination of groundwater and surface water by volatile organic compounds (VOCs) is a problem at many industrial sites. VOCs are present in effluents from industries such as petroleum refineries and chemical plants. Styrene, which is toxic to humans, is one of the VOCs used in large quantities as an important industrial material; consequently, it is present in many industrial effluents. In this study, the potential of pervaporation process for treating styrene contaminated water, simulated a petrochemical wastewater, was evaluated by using a flat-thin composite polydimethylsiloxane (PDMS) membrane. The experiments were carried out for 6 h. The influences of different experimental conditions such as temperature, initial concentration of styrene, permeate-side pressure, feed flowrate and membrane thickness on the styrene removal efficiency was investigated. Result showed that during the first hour of the experiments about 65% of styrene was removed. The results of experiments confirm that pervaporation process applied to the separation of organic compound from water and wastewater is very promising method. PDMS membrane showed very good properties in the separation of styrene, reaching 99% removal of that compound.
Removal of organic vapors from air by selective membrane permeation Journal of Membrane Science Volume 36, 1988, Pages 363–372 H. Paul, et al.
Abstract Many industrial processes such as printing, metal cleaning or painting produce waste air streams containing low concentrations of organic solvents, such as acetone, toluene, perchloroethane, xylene, etc. The total value of the solvent lost with the waste air is considerable. In addition, these solvents represent a significant pollution problem, and in recent years several procedures for recovering solvents from air, such as carbon adsorption, incineration, etc. have been introduced in the industry. All of them show some draw-backs in terms of efficiency, reliability and costs. In this paper a membrane process is described, which provides an attractive alternative to the conventional methods. In a basic study the permeabilities of acetone, toluene, xylene, dichloroethane and dichloromethane through a homogeneous polydimethylsiloxane (PDMS) membrane have been determined using a pressure difference between 400 and 1000 mbar as driving force. The selectivity of the membrane for the various solvents and their dependence on the solvent concentration in a mixture with nitrogen were studied. The selectivities of the PDMS membrane for the solvent/nitrogen mixtures were in the range of S = 70-160, depending on the solvent and its concentration in the feed mixture. Based on these data a pilot plant process has been designed in which a solvent is recovered from a waste air stream and the depleted air is recycled into the waste air producing process. The solvent which has permeated the membrane is recovered as a liquid by condensation. Thus, complete recycling of air and solvents is possible. In a cost analysis it has been demonstrated that the membrane process is indeed an attractive alternative to conventional air cleaning techniques.
Solvent recovery from air Journal of Membrane Science Volume 36, 1988, Pages 477–488 K. Kimmerle, et al.
Abstract In industrial processes such as drying, glueing, and coating, huge air streams become loaded with solvents. For economical and environmental reasons these solvents should be reused. Composite membranes produced in hollow fiber form using polydimethylsiloxane as the selective barrier are tested to recover these solvents. A hollow fiber module was built into a closed loop and the solvent was removed from the air-acetone stream inside. To characterize the essential process parameters, stationary and non-stationary experiments were performed and their results compared. The economical evaluation shows a recovery cost of 0.41 DM/kg solvent, which leads to a time for the return of investment of 1.7 years.
A flow-through passive dosing system for continuously supplying aqueous solutions of hydrophobic chemicals to bioconcentration and aquatic toxicity tests Chemosphere 86 (2012) 593–599 Margaretha Adolfsson-Erici et al.
Abstract A continuous supply of water with defined stable concentrations of hydrophobic chemicals is a requirement in a range of laboratory tests such as the OECD 305 protocol for determining the bioconcentration factor in fish. Satisfying this requirement continues to be a challenge, particularly for hydrophobic chemicals. Here we present a novel solution based on equilibrium passive dosing. It employs a commercially available unit consisting of 16000 polydimethylsiloxane (PDMS) tubes connected to two manifolds. The chemicals are loaded into the unit by repeatedly perfusing it with a methanol solution of the substances that is progressively diluted with water. Thereafter the unit is perfused with water and the chemicals partition from the unit into the water. The system was tested with nine chemicals with logKOW ranging from 4.1 to 6.3. The aqueous concentrations generated were shown to be largely independent of the water flow rate, and the unit to unit reproducibility was within a factor of 2. In continuous flow experiments the aqueous concentrations of most of the study chemicals remained constant over 8 d. A model was assembled that allows prediction of the operating characteristics of the system from the logKOW or PDMS/water partition coefficient of the chemical. The system is a simple, safe, predictable and flexible tool that generates stable aqueous concentrations of hydrophobic chemicals.
Siloxane removal using silicone–rubber membranes Separation and Purification Technology, Volume 89, 22 March 2012, Pages 234–244 Marc Ajhar et al.
Abstract Landfill and digester gas purification processes usually incorporate the removal of volatile methylsiloxanes (VMS). State-of-the-art technology is adsorption on activated carbon. This paper investigates a potential alternative: membranes. The permeabilities of common VMS in a commercially available polydimethylsiloxane (PDMS) membrane are determined as a function of temperature. A synthetic biogas mixture containing silicon in landfill gas-typical concentrations is purified in 3-end and 4-end operation. The results are presented using dimensionless numbers to facilitate upscaling. In general, PDMS can be used for siloxane removal, especially in 4-end operation using ambient air as sweep gas, where energy demand is significantly lower than in 3-end. However, depending on the desired degree of purification, methane losses of approximately 7% must be accepted. Only alternative membrane materials with higher carbon dioxide–methane selectivities have the potential for lower methane losses.
PPB-level detection of Halogenated Hydrocarbons in Drinking Water with an Electrochemical Sensor Abstract #2078, 219th ECS Meeting, © 2011 The Electrochemical Society J. R. Stetteret al.
MedArray provides its PermSelect® membrane modules for VOC removal and recovery directly to researchers, and to industry through original equipment manufacturers (OEM’s) who are interested in integrating VOC control solutions in their equipment and environment. We can also customize membrane modules to your specific application. Contact us to discuss your custom application.