A bioreactor typically refers to a device or system that supports a biologically active environment. A bioreactor can be a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. A cell culture bioreactor refers to a device or system meant to grow cells or tissues in the context of cell culture.
Bioreactor processes can either be aerobic or anaerobic, but successful processes require effective management and quality control of dissolved gas composition (O2 and CO2) and pH within the media, whether in a stirred tank or perfusion bioreactor. In many cases it is desirable to add gases such as oxygen or to eliminate CO2 to maintain the bio-processes viable, and to enable high production rates. Gases are typically contained in the bioreactor as dissolved oxygen (DO) and dissolved CO2 (DCO2) in the media or broth. Optimal oxygenation is perhaps the most difficult task to accomplish. Oxygen is poorly soluble in water and even less in fermentation broths. Oxygen transfer is usually helped by agitation, which is also needed to mix nutrients and to keep the media homogeneous. There are, however, limits to the speed of agitation, due both to high power consumption and to the damage to organisms caused by excessive paddle speed or gas lift mechanisms.
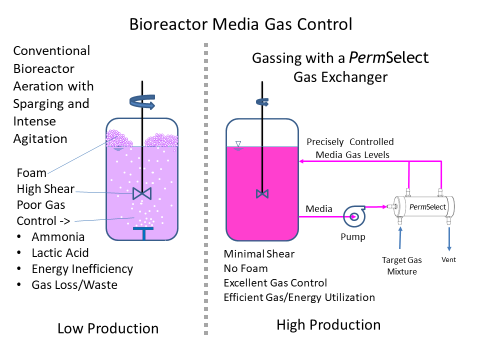
Rather than subject broths or cell culture media to higher shear stress, foaming due to sparging, or deal with increasingly large headspace and pressure requirements to oxygenate the media, MedArray's silicone hollow fiber membrane gas exchangers can regulate dissolved oxygen and CO2 (DO and DCO2) composition to meet cell culture media requirements and to achieve the desired productivity and quality control. Our membrane gas exchangers use silicone hollow fibers which are dense, without pores, and thus not susceptible to fouling. Since the gas exchange occurs via diffusion through the membrane, it does not produce bubbles and as a result, eliminates foaming within the bioreactor, in effect enabling bubble-free sparging. Additionally, silicone is inert (low extractables) and well characterized in biopharmaceutical applications. Our PermSelect® gas exchangers are ideal for controlling cell culture media dissolved O2 and CO2 composition, as well as pH, thereby meeting requirements and optimizing production and quality control. In effect our membrane performs as a highly effective oxygenator in stirred tank and perfusion bioreactors. Click here for more detailed information on our gas exchangers for cell culture bioreactors.
Our membrane modules are also ideal for pharmaceutical liquid degassing and can be sterilized by steam autoclave, gamma radiation, and other harsh chemicals. Please view our sterilization guidelines page. PermSelect membrane degassers are available in FDA compliant materials, including USP Class VI, ISO 10993-5, and FDA CFR 21 compliant materials. Prior to shipping, each membrane degasser is individually tested following a rigorous protocol outlined at our testing page. Our membrane degassers provided to bio-pharmaceutical customers are shipped with a material certificate of compliance (MCC). Contact us for details.
Warning: Membrane modules are supplied non-sterile. It is the responsibility of the end user to validate the sterility of the module as a component in their particular system to be sterilized. Please view the sterilization guidelines as an initial sterilization guide.
How do I control dissolved gases in a bioreactor using a PermSelect® membrane module?
Using a PermSelect® silicone membrane gas exchanger to control dissolved gases such as O2 and CO2 in your bioreactor is simple. There are many options depending on your specific application but the most straightforward configuration is illustrated above. A media stream is continuously drawn from a selected location in the bioreactor vessel and driven with a pump through the shell side of the PermSelect® membrane gas exchanger. A gas (such as oxygen) or mixture of gases is flown through the lumen side of the membrane gas exchanger to equilibrate the dissolved gases in the media to this gas composition. The conditioned media exiting the membrane gas exchanger is returned to the tank, thereby maintaining a desired bioreactor dissolved gas composition in the media. To remove gases from your bioreactor (such as excessive CO2 or other toxic gases or organic vapors) a vacuum may be applied to the tube side of the module.
Silicone (PDMS) membranes have been used and demonstrated to provide adequate gas control in stirred tank bioreactors. The following published articles describe the effective use silicone membranes for this purpose.
- Controlled gas exchange in whole lung bioreactors J Tissue Eng Regen Med 2018; 12:e119–e129. Alexander J. Engler Andrew V. Le Pavlina Baevova Laura E. Niklason
Abstract In cellular, tissue‐level or whole organ bioreactors, the level of dissolved oxygen is one of the most important factors requiring control. Hypoxic environments may lead to cellular apoptosis, while hyperoxic environments may lead to cellular damage or dedifferentiation, both resulting in loss of overall tissue function. This manuscript describes the creation, characterization and validation of a bioreactor system that can control oxygen delivery based on real‐time metabolic demand of cultured whole lung tissue. A mathematical model describing and predicting gas exchange within the tunable bioreactor system is developed. In addition, the inherent gas exchange properties of the bioreactor and the inherent oxygen consumption rates of native rat lungs are determined, thereby providing a quantitative relationship between system parameters and levels of dissolved oxygen. Finally, the mathematical model is validated during whole lung culture under a range of system parameters. The system presented here provides a quantitative relationship between the concentration of dissolved oxygen, tissue oxygen consumption rates, and controllable system parameters that introduce gasses into the bioreactor. This relationship not only enables the maintenance of constant levels of dissolved oxygen throughout a culture period during which cells are replicating, but also provides noninvasive and real‐time estimation of the metabolic and proliferative states of native or engineered lung tissue simply through dissolved oxygen measurements.
- Simultaneous biohydrogen production and purification in a double-membrane bioreactor system International Journal of Hydrogen Energy (Impact Factor: 3.31). 01/2015; 40(4):1690-1697. DOI: 10.1016/j.ijhydene.2014.12.002 P. Bakonyi, et al.
Abstract In this work the establishment of a double-membrane bioreactor was aimed. Initially, a continuous hydrogen fermenter was coupled with a commercial Kubota® microfiltration membrane module and the production performance of the cell-retentive design was evaluated under various hydraulic retention times. As a result, it has been observed that altering HRT influenced the rejection feature of the microfiltration module while had an inverse effect on hydrogen productivity and yield, since shortened HRTs were accompanied by gradually decreasingH2yields(HY)and progressively increasing volumetric H2production rates(HPR).The highest HY and HPR were achieved as 1.13 mol H2/mol glucose and 0.24 mol H2/L-d, respectively. Furthermore, a Permselect®(PDMS)gas separation membrane was installed to the anaerobic membrane bioreactor and its ability to separate hydrogen from the raw fermentation gaseous mixture was assessed. The highest purity hydrogen obtained in one-step purification by the PDMS module was 67.3 vol.%, which exceeds30%enrichment efficiency considering 51.3vol% H2in the feed gas. Hence, it could be concluded that the poly(dimethylsiloxane)membrane has potential to attractively concentrate biohydrogen from fermenter off-gas and may be used for in-situ product recovery.
- A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors Bioresource Technology Volume 133, April 2013, Pages 340–346 J.J. Orgill, et al.
Abstract Trickle-bed reactor (TBR), hollow fiber membrane reactor (HFR) and stirred tank reactor (STR) can be used in fermentation of sparingly soluble gasses such as CO and H2 to produce biofuels and bio-based chemicals. Gas fermenting reactors must provide high mass transfer capabilities that match the kinetic requirements of the microorganisms used. The present study compared the volumetric mass transfer coefficient (KtotA/VL) of three reactor types; the TBR with 3 mm and 6 mm beads, five different modules of HFRs, and the STR. The analysis was performed using O2 as the gaseous mass transfer agent. The non-porous polydimethylsiloxane (PDMS) HFR provided the highest KtotA/VL (1062 h−1), followed by the TBR with 6 mm beads (421 h−1), and then the STR (114 h−1). The mass transfer characteristics in each reactor were affected by agitation speed, and gas and liquid flow rates. Furthermore, issues regarding the comparison of mass transfer coefficients are discussed.
Contact an applications engineer, or call +1 (734) 769-1066 to discuss your particular bioreactor gas control needs. MedArray provides its PermSelect® membrane modules to researchers, and to industry through original equipment manufacturers (OEM’s) who are interested in integrating gas control solutions in their bioreactors. We can also customize membrane modules to your specific application. Contact us to discuss your custom application.