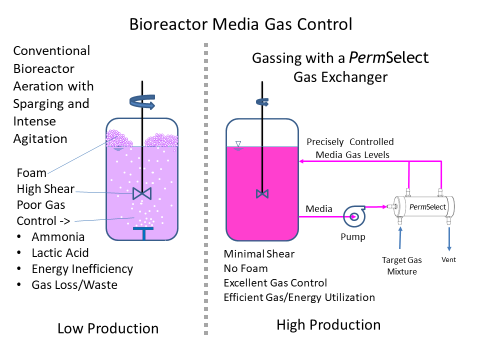
The most essential scale-up criteria for bioreactors used for cell culture is effective control of dissolved O2 and CO2 within the bioreactor media. As densities increase, aeration in cell culture bioreactor media, i.e., gassing, oxygenation and CO2 stripping requirements, go up dramatically. Rather than subject cells to a bioreactor control system using aeration with agitation and high shear stress from paddles, foaming due to sparging and agitation, or deal with increasingly large headspace to oxygenate the media, our PermSelect® silicone membrane gas exchangers can provide optimal aeration with increased oxygen mass transfer (high kLa) and CO2 stripping rates (kLaCO2) to produce the scaled-up levels of dissolved O2 (DO) and dissolved CO2 (DCO2). Thus our gas exchangers effectively produce increased aeration with reduced agitation enabling bioreactor kLa scale-up. Moreover, our gas exchangers effectively enable a CO2 stripping bioreactor.
Many mammalian cells such as the Chinese hamster ovary cells, or CHO cells, are highly sensitive to shear from agitation and aeration systems in cell culture. Increasing cell densities and scale-up in stirred tank bioreactors presents new issues associated with cell shear damage including high energy dissipation in the immediate impeller zones, small gas bubble burst, and high gas entrance velocity from a sparger. Thus the negative effect of increased agitation and shear must be considered in CHO gassing approaches for bioreactor scale-up. Similarly, scale-up in perfusion bioreactors systems used for monoclonal antibody production and the continuous processing of primary stem cells can be limited by poor media oxygenation and gas exchange which can result in detrimental levels of the inhibitory metabolites lactic acid and ammonia.
Our membrane gas exchangers are ideal for media gas control in cell culture bioreactors, in effect acting as lungs supplying O2 and removing CO2 from the bioreactor media. Figure 1 below illustrates a comparison between traditional sparging with intense agitation (left panel), and using a PermSelect gas exchanger to oxygenate the cell culture media (right panel). As shown in the diagram, a media stream is continuously drawn from a selected location in the bioreactor vessel and driven with a pump through the shell side of the PermSelect® gas exchanger. A gas (such as air, oxygen) or mixture of gases is flown through the lumen side of the gas exchanger to equilibrate the dissolved gases in the media to this gas composition. The oxygenated media exiting the gas exchanger is returned to the bioreactor vessel, thereby maintaining a desired bioreactor dissolved O2 and CO2 composition in the media. Other gases in the bioreactor (such as excessive CO2, ammonia, or other toxic gases) are stripped from the media.
Note that the problems associated with sparging and agitation are eliminated with the PermSelect gas exchanger performing as the "lung" to the bioreactor. However, just as with the native lungs, it is necessary to flow media through the gas exchanger at a sufficient flowrate to pick-up and carry enough dissolved gases to meet the metabolic requirements of the bioreactor.
To properly size the gas exchanger to the metabolic requirements of the bioreactor it is necessary to know the cell count (or the cell density and volume) and the cell consumption rate, or target kLa. For example, the oxygen consumption rate for typical recombinant mammalian cell lines used in bioproduction is on the order of 0.003 - 0.006 mg O2/106Cells/hr, and cell densities are in the range of 100 x 106 Cells/ml. Thus assuming a oxygen consumption rate of 0.005 mg O2/106Cells/hr, the oxygen uptake rate (OUR) is calculated as:
- OUR = (oxygen consumption rate) x (cell density) x (volume)
- OUR = (0.005 mg O2/106 Cells/hr) x (100·106 Cells/ml) x (volume)
- Assuming a bioreactor volume of 2 liters (2000 ml)
- OUR = (0.5 mg O2/ml/hr) x (2000 ml)
- OUR = 1000 mg O2/hr.
Thus the gas exchanger must be properly sized to provide an oxygen transfer rate (OTR) of at least 1000 mg O2/hr. Figure 2 below provides a graph of the OTR for PermSelect gas exchangers at various flowrates. Upon finding the target OTR on the vertical scale, we find that there are three gas exchangers that can provide the required OTR at different flowrates: PDMSXA-2.1 at 3 L/min, PDMSXA-1.0 at 4.3 L/min, and PDMSXA-7500 at 5.4 L/min. The selection of gas exchanger to use will depend on bioreactor configuration (i.e., draw/return sites), pump capacity, and desired mixing of returned oxygenated media.
PermSelect gas exchangers can also be sized based on a known target kLa for a bioreactor volume. The table below provides the values of the mass transfer coefficient kLa for each PermSelect® gas exchanger used in conjunction with various bioreactor tank sizes. The value of kLa on the graphs was estimated using measured oxygen transfer rates (OTR) for the gas exchanger, and assuming the media is drawn from the bioreactor tank at zero oxygen content and driven in a single pass through the gas exchanger to a second tank at the indicated flow, with air flowing (sweep) at the same rate as the media. Note that using oxygen instead of air as a sweep flow will result in higher kLa.
Our gas exchangers use a silicone gas exchange membrane which is dense, without pores, and thus not susceptible to fouling or pore wet-out. Since the gas transfer occurs via diffusion through the membrane, it does not produce bubbles and as a result, eliminates foaming and shear within the culture vessel, in effect enabling bubble-free sparging. Additionally, silicone is inert (low extractables) and well characterized in biopharmaceutical applications. Our PermSelect® gas exchangers can be used with batch fed and perfusion bioreactors as well as with single use and disposable bioreactors. Moreover, they can be used in conjunction with existing aeration methods (i.e., to supplement oxygen and CO2 transfer), or as a replacement of existing and problematic methods.
Our membrane gas exchangers are also ideal for pharmaceutical liquid degassing and can be sterilized by steam autoclave (including SIP), gamma radiation, EtO, and other harsh chemicals. Please view our sterilization guidelines page. PermSelect® membrane degassers are available in FDA compliant materials, including USP Class VI, ISO 10993-5, and FDA CFR 21 compliant materials. Prior to shipping, each membrane degasser is individually tested following a rigorous protocol outlined at our testing page. Our membrane degassers provided to bio-pharmaceutical customers are shipped with a material certificate of compliance (MCC). Contact us for details.
Warning: Membrane modules are supplied non-sterile. It is the responsibility of the end user to validate the sterility of the module as a component in their particular system to be sterilized. Please view the sterilization guidelines as an initial sterilization guide.
How do you scale-up a bioreactor using a PermSelect® gas exchanger?
Using a PermSelect® silicone membrane gas exchanger for scale-up in a bioreactor is simple. There are many options depending on your specific application but the most straightforward configuration is illustrated in Figure 1 above (right panel). A media stream is continuously drawn from a selected location in the bioreactor vessel and driven with a pump through the shell side of the PermSelect® gas exchanger. A gas (such as air, oxygen) or mixture of gases is flown through the lumen side of the gas exchanger to equilibrate the dissolved gases in the media to this gas composition. The oxygen enriched media exiting the gas exchanger is returned to the bioreactor vessel, thereby maintaining a desired bioreactor dissolved O2 and CO2 composition in the media. Other gases in the bioreactor (such as excessive CO2, ammonia, or other toxic gases) are stripped from the media.
Please view the videos below explaining how our membrane gas exchangers can be used to control dissolved gases in cell culture bioreactors.
The following published articles describe the effective use silicone membranes for this purpose.
Micro-Bioreactors in Space: Case Study of a Yeast (Saccharomyces cerevisiae) Bioreactor With a Non-Invasive Monitoring Method Frontiers in Space Technologies, VOLUME 2, 2022 Granata Tim, Rattenbacher Bernd, John Gernot
ABSTRACT Bioreactors in space have applications from basic science to microbial factories. Monitoring bioreactors in microgravity has challenges with respect to fluidics, aeration, sensor size, sample volume and disturbance of medium and cultures. We present a case study of the development of small bioreactors and a non-invasive method to monitor dissolved oxygen, pH, and biomass of yeast cultures. Two different bioreactor configurations were tested for system volumes of 60 ml and 10.5 ml. For both configurations, the PreSens SFR vario, an optical sensor array, collected data autonomously. Oxygen and pH in the cultures were monitored using chemically doped spots, 7 mm in diameter, that were fixed to the bottom of sampling chambers. Spots emitted a fluorescent signal for DO and pH when reacted with oxygen molecules and hydrogen ions, respectively. Biomass was sensed using light reflectance at centered at 605 nm. The, optical array had three light detectors, one for each variable, that returned signals that were pre- and post-calibrated. For heterotrophic cultures requiring oxygen and respiring carbon dioxide, a hollow fiber filter, in-line with the optical array, oxygenated cells and remove carbon dioxide. This provided oxygen levels that were sufficient to maintain aerobic respiration for steady state conditions. Time series of yeast metabolism in the two bioreactors are compared and discussed. The bioreactor configurations can be easily be modified for autotrophic cultures such that carbon dioxide is enhanced and oxygen removed, which would be required for photosynthetic algal cultures.
Critical parameters and procedures for anaerobic cultivation of yeasts in bioreactors and anaerobic chambers. FEMS Yeast Research, Volume 21, Issue 5, August 2021, Christiaan Mooiman, Jonna Bouwknegt, Wijb J C Dekker, Sanne J Wiersma, Raúl A Ortiz-Merino, Erik de Hulster, Jack T Pronk
ABSTRACT. All known facultatively fermentative yeasts require molecular oxygen for growth. Only in a small number of yeast species, these requirements can be circumvented by supplementation of known anaerobic growth factors such as nicotinate, sterols and unsaturated fatty acids. Biosynthetic oxygen requirements of yeasts are typically small and, unless extensive precautions are taken to minimize inadvertent entry of trace amounts of oxygen, easily go unnoticed in small-scale laboratory cultivation systems. This paper discusses critical points in the design of anaerobic yeast cultivation experiments in anaerobic chambers and laboratory bioreactors. Serial transfer or continuous cultivation to dilute growth factors present in anaerobically pre-grown inocula, systematic inclusion of control strains and minimizing the impact of oxygen diffusion through tubing are identified as key elements in experimental design. Basic protocols are presented for anaerobic-chamber and bioreactor experiments.
Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae. Fermentation 2021, 7, 282. Granata, T.; Follonier, C.; Burkhardt, C.; Rattenbacher, B.
Abstract. Maintaining steady-state, aerobic cultures of yeast in a bioreactor depends on the configuration of the bioreactor system as well as the growth medium used. In this paper, we compare several conventional aeration methods with newer filter methods using a novel optical sensor array to monitor dissolved oxygen, pH, and biomass. With conventional methods, only a continuously stirred tank reactor configuration gave high aeration rates for cultures in yeast extract peptone dextrose (YPD) medium. For filters technologies, only a polydimethylsiloxan filter provided sufficient aeration of yeast cultures. Further, using the polydimethylsiloxan filter, the YPD medium gave inferior oxygenation rates of yeast compared to superior results with Synthetic Complete medium. It was found that the YPD medium itself, not the yeast cells, interfered with the filter giving the low oxygen transfer rates based on the volumetric transfer coefficient (KLa). The results are discussed for implications of miniaturized bioreactors in low-gravity environments.
A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors Silicone (PDMS) membranes have been used and demonstrated to provide adequate oxygen supply to high density cell cultures and to aid in stirred tank bioreactors. Bioresource Technology Volume 133, April 2013, Pages 340–346 J.J. Orgill, et al.
Abstract Trickle-bed reactor (TBR), hollow fiber membrane reactor (HFR) and stirred tank reactor (STR) can be used in fermentation of sparingly soluble gasses such as CO and H2 to produce biofuels and bio-based chemicals. Gas fermenting reactors must provide high mass transfer capabilities that match the kinetic requirements of the microorganisms used. The present study compared the volumetric mass transfer coefficient (KtotA/VL) of three reactor types; the TBR with 3 mm and 6 mm beads, five different modules of HFRs, and the STR. The analysis was performed using O2 as the gaseous mass transfer agent. The non-porous polydimethylsiloxane (PDMS) HFR provided the highest KtotA/VL (1062 h−1), followed by the TBR with 6 mm beads (421 h−1), and then the STR (114 h−1). The mass transfer characteristics in each reactor were affected by agitation speed, and gas and liquid flow rates. Furthermore, issues regarding the comparison of mass transfer coefficients are discussed.
Controlled gas exchange in whole lung bioreactors J Tissue Eng Regen Med 2018; 12:e119–e129. Alexander J. Engler Andrew V. Le Pavlina Baevova Laura E. Niklason
Abstract In cellular, tissue‐level or whole organ bioreactors, the level of dissolved oxygen is one of the most important factors requiring control. Hypoxic environments may lead to cellular apoptosis, while hyperoxic environments may lead to cellular damage or dedifferentiation, both resulting in loss of overall tissue function. This manuscript describes the creation, characterization and validation of a bioreactor system that can control oxygen delivery based on real‐time metabolic demand of cultured whole lung tissue. A mathematical model describing and predicting gas exchange within the tunable bioreactor system is developed. In addition, the inherent gas exchange properties of the bioreactor and the inherent oxygen consumption rates of native rat lungs are determined, thereby providing a quantitative relationship between system parameters and levels of dissolved oxygen. Finally, the mathematical model is validated during whole lung culture under a range of system parameters. The system presented here provides a quantitative relationship between the concentration of dissolved oxygen, tissue oxygen consumption rates, and controllable system parameters that introduce gasses into the bioreactor. This relationship not only enables the maintenance of constant levels of dissolved oxygen throughout a culture period during which cells are replicating, but also provides noninvasive and real‐time estimation of the metabolic and proliferative states of native or engineered lung tissue simply through dissolved oxygen measurements.
Oxygenation of intensive cell-culture system Applied Microbiology and Biotechnology Volume 43, Number 6, 1028-1033 A. N. Emery, et al.
Abstract The abilities of various methods of oxygenation to meet the demands of high-cell-density culture were investigated using a spin filter perfusion system in a bench-top bioreactor. Oxygen demand at high cell density could not be met by sparging with air inside a spin filter (oxygen transfer values in this condition were comparable with those for surface aeration). Sparging with air outside a spin filter gave adequate oxygen transfer for the support of cell concentrations above 107 ml–1 in fully aerobic conditions but the addition of antifoam to control foaming caused blockage of the spinfilter mesh. Bubble-free aeration through immersed silicone tubing with pure oxygen gave similar oxygen transfer rates to that of sparging with air but without the problems of bubble damage and fouling of the spin filter. A supra-optimal level of dissolved oxygen (478% air saturation) inhibited cell growth. However, cells could recover from this stress and reach high density after reduction of the dissolved oxygen level to 50% air saturation.
Contact an applications engineer, or call +1 (734) 769-1066 to discuss your particular cell culture bioreactor gas control needs. MedArray provides its PermSelect® membrane modules to researchers, and to industry through original equipment manufacturers (OEM’s) who are interested in integrating gas control solutions in their cell culture bioreactors. We can also customize membrane modules to your specific application. Contact us to discuss your custom application.